Introduction
Lithium-ion batteries are used in a wide range of things such as smartphones, computers, robots, and most other electronics due to their ability to store a high power capacity.
Lithium-ion batteries can charge very quickly, are long-lasting, and currently provide the best technology to power electronics.
A lithium-ion battery is made up of lithium ions moving between the negative (anode) and positive (cathode) electrodes through electrolytes.


How do Lithium-Ion Batteries Work?
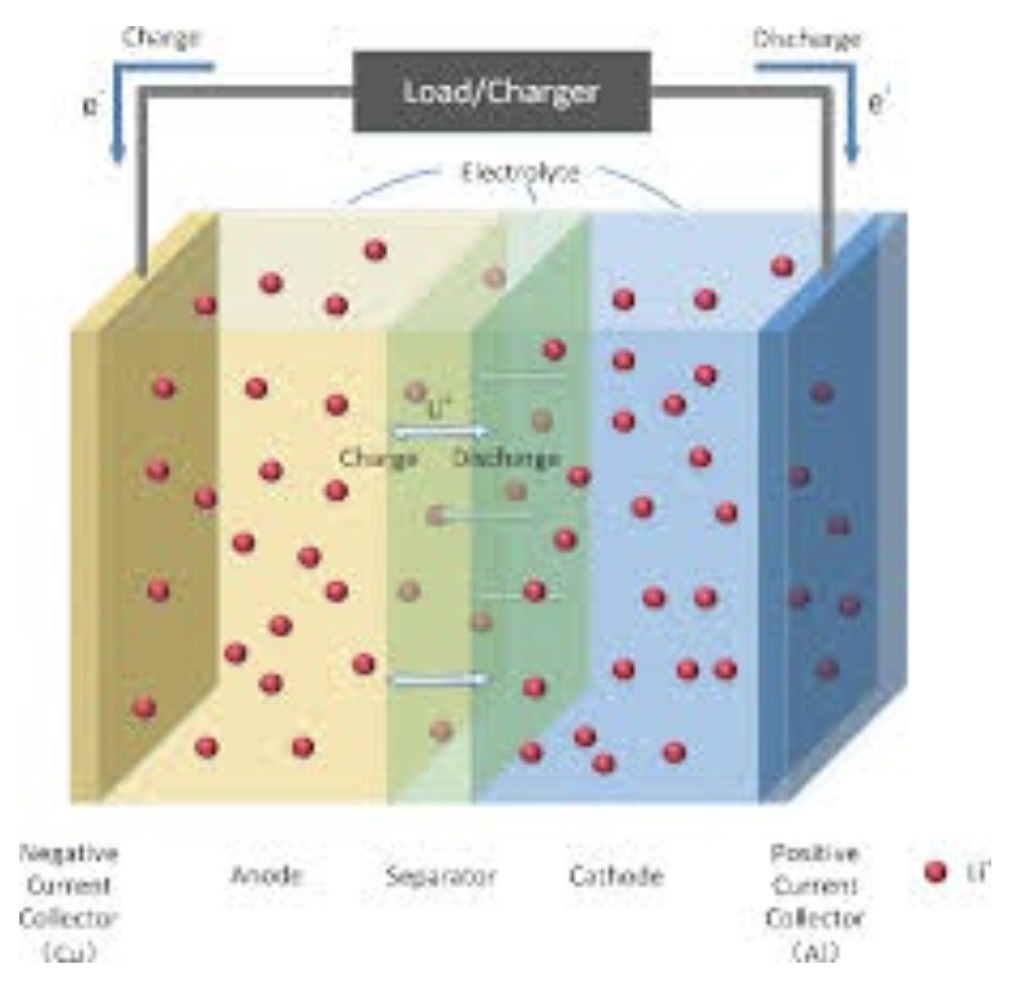
A lithium-ion battery is made of mainly three sections which are the positive electrode (blue), the negative electrode (yellow), and the electrolyte in between them (green). The positive electrode is made of lithium cobalt oxide (LiCoO2) and sometimes made from lithium iron phosphate (LiFePO4) for newer batteries. The negative electrode is commonly made from carbon. The electrolyte varies from one type of battery to another. All lithium-ion batteries work typically in the same way.
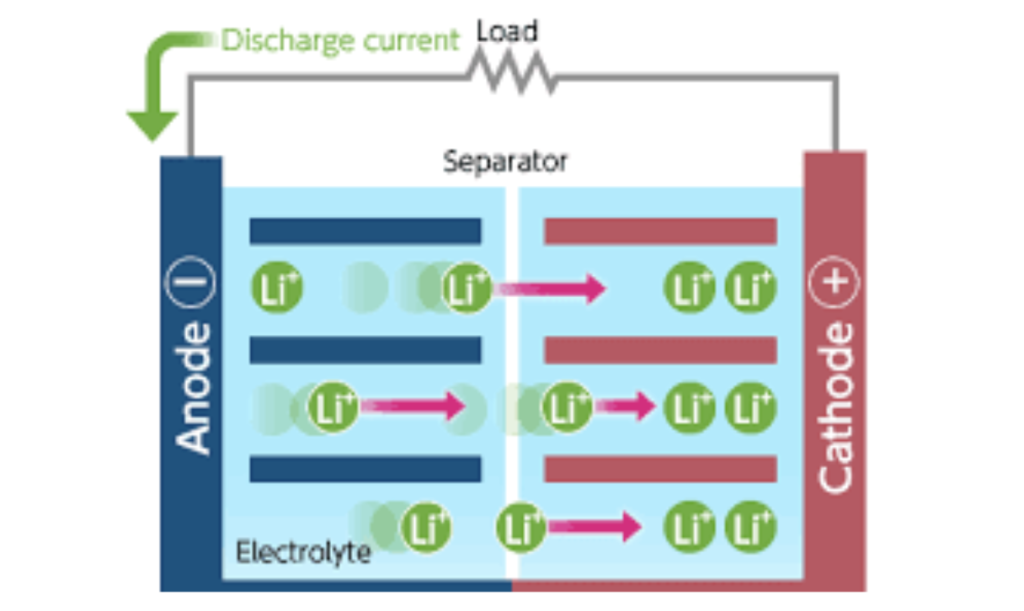
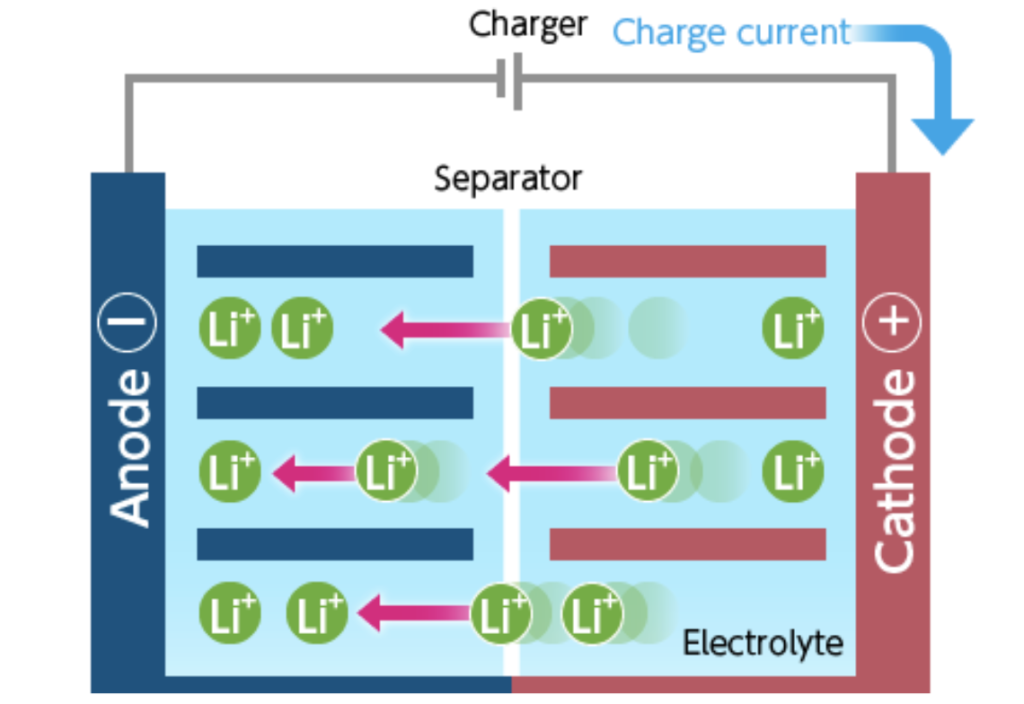
The positive electrode delivers the lithium ions to the negative electrode through the electrolytes when the battery is charging. Lithium ions that transferred while charging remain in the negative electrode. The battery took in and stored energy in this process.
When the battery is discharging, the lithium ions move from the negative electrode back to the positive electrode through the electrolyte producing the energy that powers the battery. Electrons while charging and discharging will flow in the opposite direction to the ions around the outer circuit and will not flow through the electrolyte.
The movement of ions (electrolyte) and electrons (outer circuit) are strongly correlated. If either of them stops, the other stops too. If the battery completely discharges, ions stop moving through the electrolyte. Thus, electrons can’t move through the outer circuit and lose power. Similarly, if you switch off the powering battery, the flow of electrons will stop, and the flow of ions will stop too.
Advantages of Lithium-Ion Batteries
1 Light
They are lighter than other types of metals used for rechargeable batteries of the same size. The electrodes are made of lightweight lithium and carbon.
2 Reactive
Lithium easily loses its electrons from the valence shell and allows the battery’s current to flow very well.
Lithium-ion Battery vs. NiMH battery:
- Lithium-ion battery: store 150 watt-hours of electricity in 1 kilogram of battery
- NiMH battery (Nickel-metal hydride): store 60~70 watt-hours of electricity in 1 kilogram of battery
- (Lead-acid batteries: 1kg lithium-ion batteries = 6 kilograms of lead-acid batteries)
- A lithium-ion battery pack loses only about 5 percent of its charge per month
- NiMH batteries lose 20 percent of their charge per month
3 Rechargeable
Lithium-ion batteries are rechargeable as lithium ions, and electrons move easily back into negative electrodes.
Disadvantages of Lithium-Ion Batteries
1 Fast Degrading
Lithium-ion batteries start degrading as soon as they leave the factory. They will only last two or three years from the date of manufacture.
2 Sensitive to High Temperature
Heat causes the battery packs to degrade even faster than they usually would.
3 Ruined when completely discharged
4 Expensive
A lithium-ion battery pack must have an on-board computer to manage the battery, making them even more expensive than they already are.
5 Burst
Some people might have seen the news of phones bursting. Although it is a small chance, if a lithium-ion battery pack fails, it will burst into flame.

[1] Apple iPhone Battery Replacement (Repair Included). 4 Oct. 2020, www.fixfactory.ca/shop/apple-iphone-battery-replacement-repair-included/.
[2] LMP Battery MacBook Pro. 4 Oct. 2020 https://lmp-adapter.com/product/lmp-battery-macbook-pro-17-alu-unibody-2/.
[3] Jianan Zhang. Schematic of the Lithium-ion battery. 4 Oct. 2020 https://www.researchgate.net/figure/Schematic-of-the-Lithium-ion-battery_fig2_324929541.
[4][5] How lithium-ion batteries work. 4 Oct. 2020, https://www.scib.jp/en/product/sip/download/batteryschool/episode1.htm.
[8] https://electronics.howstuffworks.com/everyday-tech/lithium-ion-battery.htm.