History of Fireworks
Historians believe that fireworks were invented by the Chinese in the second century. Everything started off by an accident when people noticed that bamboo sticks in their bonfires exploded with a bang. This was caused due to the fast-growing bamboo trapping air inside its stalks. A huge reaction with light and sound was caused when oxygen came in contact with the fire.
Chinese alchemists made an improvement from this when they mixed together sulfur, potassium nitrate, arsenic disulfide, and honey. This was the first form of gunpowder. When the fire was set, the explosion was caused by the sulfur and honey as they acted as the energy source for the reaction. The potassium nitrate provided the oxygen for the reaction.
Chinese packed the new gunpowder into bamboo shoots and threw the shoots into a fire. This was the first firework. Everything started from a bamboo explosion and it has evolved gradually into what we have now today.

Structure of Firework Shell
![Screen Shot 2020-09-26 at 12.39.55 [13] Structure of Firework Shell](https://ergo-science.com/wp-content/uploads/elementor/thumbs/Screen-Shot-2020-09-26-at-12.39.55-ow0lrr05xfplwerh3rm260ef3l5h0jtbmf5iy7h3dc.jpg)
![Screen Shot 2020-09-26 at 12.40.30 [14] Inside the Firework Shell](https://ergo-science.com/wp-content/uploads/elementor/thumbs/Screen-Shot-2020-09-26-at-12.40.30-ow0lsi9hfmqx93nvole8obisbrf47rtje62lv8cocw.jpg)
A firework shell is made of heavy paper or cardboard that holds mainly the “lift charge”, the “bursting charge”, and the “stars”.
Lift charge is found at the bottom of the firework shell and is concentrated with gun powder (Charcoal, sulfur, and potassium nitrate). The gunpowder is tightly packed in the lift charge and when the fuse is lit by the fire, it creates a powerful force that lifts it up to the air at a very fast speed which can send it as high as 300 meters.
Stars are balls made up of fuels, oxidizers, and colorants. These stars measure about 3 to 4 centimeters in diameter. Stars are what makeup what people eventually see in the sky. Each star makes one dot in the fireworks explosion. The stars can be arranged within the firework shell to create shapes such as a smiley face and a Saturn. Moreover, hundreds of stars can be used in a single firework shell.
The bursting charge found in the middle of the firework shell is also made of gun powder. The bursting charge creates the heat to activate the stars that surround the bursting charge. This allows the stars to explode outward from the shell. The bursting charge helps the fireworks explode in the air.
Oxidizers in Firework Shells
Oxidizers are in need to create fireworks since plenty of oxygen is necessary in order to facilitate the burn. Oxidizers release excess oxygen to make a better explosion. Potassium nitrate, potassium perchlorate, and strontium nitrate are often used for oxidizers in fireworks.
Chemical Reactions in Fireworks
Combustion
Combustion occurs when the flame from the firework shell’s fuse comes into contact with the gun powder. This causes potassium nitrate, charcoal, and sulfur to combine. The combustion is highly exothermic, or heat-producing which allows fireworks to force out the heat and gas from the bottom of the firework shell, shooting the firework upwards into the sky.
Oxidation
The fuse reaches a part filled with an oxidizing agent and stars when the firework reaches its highest point in the sky. The common oxidizing agents, nitrates, chlorates, and perchlorates are the oxidizers of the firework. The heat and gas formed from combustion react with the oxidizing agents, or the oxidizers in order to produce oxygen for the rapid combustion of the stars.
Energy Absorption / Emission
The oxygen that formed during oxidation reacts with the elements in the stars, which are oxidizing agents, a fuel, a metal-containing colorant, and a binder and produces a hot, rapidly expanding gas. The atoms in this rapidly expanding gas absorb the energy produced in the reaction. This causes their electrons to move from their stable state to an excited energy state. When the electrons return back to their stable state, they emit energy in the form of light.
Color of Fireworks
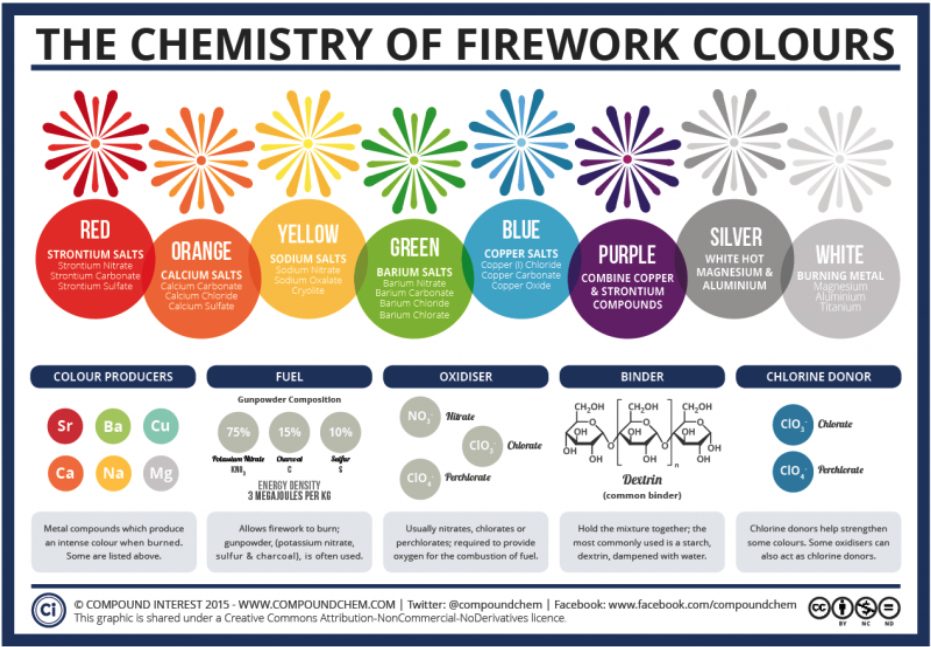
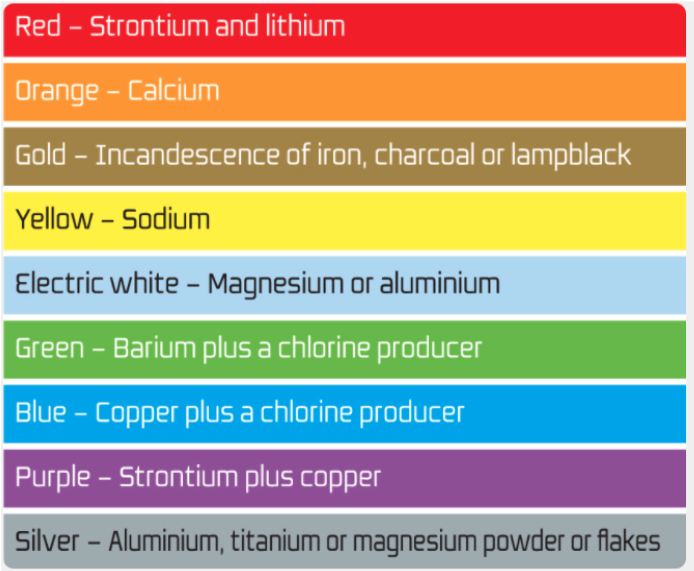
The colors of fireworks are created by the use of metal salts. They are different from the salt that we usually consume. Metal salts used for fireworks are mainly found from groups 1 and 2 from the periodic table, which are alkali metals and alkaline-earth metals. Metal salts are used for making the color of fireworks since these compounds produce intense colors when they are burned. When the metal salts are heated, their atoms absorb energy and get excited. As the electrons come down from the excited state to the stable state, they release energy about 200 kilojoules per mole, in the form of light. Different chemicals produce different amounts of energy, creating different colors. For example, strontium carbonate produces red fireworks, calcium chloride produces orange fireworks, Sodium nitrate produces yellow fireworks, barium chloride produces green fireworks, and copper chloride produces blue fireworks. Purple fireworks are produced by the mixture of strontium which produces the color red and copper which produces the color blue like how we mix blue and red paints to produce the color, purple.
[1] Bradford, Alina. “History of Fireworks.” LiveScience, Purch, 30 Aug. 2018, <https://www.livescience.com/63468-fireworks-history.html.>
[2] Brain, Marshall. “How Fireworks Work.” HowStuffWorks Science, HowStuffWorks, 30 June 2020, <https://science.howstuffworks.com/innovation/everyday-innovations/fireworks.htm.>
[3] Credits Erica K. Brockmeier Write, et al. “The Chemistry behind Fireworks.” Penn Today, 1 Jan. 1970, <https://penntoday.upenn.edu/news/chemistry-behind-fireworks.>
[4] Grush, Loren. “The Chemistry behind a Firework Explosion.” The Verge, The Verge, 3 July 2015,
<www.theverge.com/2015/7/3/8886697/the-chemistry-behind-a-firework-explosion.>
[5] “How Do Fireworks Get Their Colors?” EarthSky, <https://earthsky.org/human-world/how-do-fireworks-get-their-vibrant-colors#:~:text=Metal%20salts%20commonly%20used%20in,copper%20chloride%20(blue%20fireworks).>
[6] “How Fireworks Work: Firework Science.” Explain That Stuff, 13 Apr. 2020, <www.explainthatstuff.com/howfireworkswork.html.>
[7] Study.com, <https://study.com/academy/lesson/how-do-fireworks-work-lesson-for-kids.html.>
[8] Team, How It Works. “How Do Fireworks Explode?” How It Works, 5 Nov. 2018, <www.howitworksdaily.com/how-do-fireworks-explode/.>
[9] “The Chemistry of Fireworks.” Compound Interest, 31 Dec. 2019, <www.compoundchem.com/2013/12/30/the-chemistry-of-fireworks/.>
[10] “The Chemistry of Fireworks: Reactions Science Videos.” American Chemical Society, <www.acs.org/content/acs/en2/pressroom/reactions/videos/2014/the-chemistry-of-fireworks.html.>
[11] Wallulis, Karl. “Simple Chemical Reactions in Fireworks.” Sciencing, 2 Mar. 2019, <https://sciencing.com/simple-chemical-reactions-fireworks-7502150.html.>
[12] Porter, Toby. “Pollution Surge Caused by Garden Bonfires during Coronavirus Lockdown.” South London News, 31 Mar. 2020, <londonnewsonline.co.uk/pollution-surge-caused-by-garden-bonfires-during-coronavirus-lockdown/.>
[13] “Inside Fireworks.” a J And J Presentation – Home, <allfireworks.weebly.com/inside-fireworks.html. >
[14] https://www.youtube.com/watch?v=X8dQPLSIYVE&t=51s
[15] “Fukuoka Fireworks Guide 2020.” Fukuoka Now, 22 July 2020, <www.fukuoka-now.com/en/fukuoka-fireworks-guide/. >
