Introduction
Aluminum is used everywhere: from airplanes and batteries to window frames and house decor. Perhaps you might even use it to wrap your steak after a barbeque.
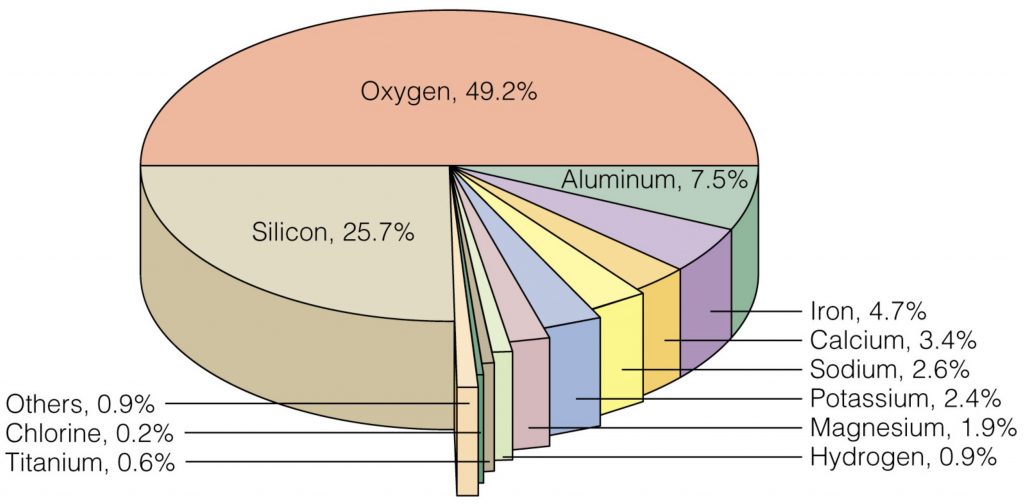
Consequently, it might be surprising for one to hear that Aluminum used to be more valuable than gold, even being the most expensive metal on earth. Can’t get your head around this? Well, during the 1800s, Napoleon III demonstrated the nobility of aluminum when he served his state dinners on aluminum plates while the lower-ranked guests had their food on silver and gold. Clearly, aluminum was treated specially, but why was this so? Aluminum is and always was the most abundant metal in the earth’s crust, and it definitely doesn’t have a cool look to it (it is shiny though).
![Charles Martin Hall Charles Martin Hall [3]](https://ergo-science.com/wp-content/uploads/elementor/thumbs/Screen-Shot-2020-08-04-at-17.04.12-otgo2p6zcy9j4wo201w0ifc0nfzyu3ppgyqu9i8wck.jpg)
Well, it has to do with the difficulty of extracting Aluminum from its ores. See, Aluminum isn’t just sitting there in the crust; it exists mainly in the form of minerals such as Alumina (Al2O3) and Bauxite (which is mainly Al(OH)3), along with other compounds. And unlike other metal ores like that of Iron, simply heating Aluminum ores doesn’t yield the metal. It was only until 1886 that the mass production of Aluminum became economically feasible, thanks to two chemists–Charles Hall and Paul Héroult–and a technique called electrolysis.
Brief History of Aluminum
- Alum (one of the Aluminum ores) was known to humans since 500 BC and was used in medicine and as a dye.
- By the middle ages, alum was a common dye and was often traded internationally.
- Aluminum metal was first discovered (isolated) in 1825 by Hans Christian Ørsted
- Shortly after its discovery, the price of Aluminum exceeded that of Gold.
- The first industrial production was succeeded by a French chemist Henry Deville in 1856. This was done by treating aluminum ores with sodium but this was still ineffective since sodium was/is expensive
- In 1886, Charles Hall and Paul Héroult developed the procedure for the production of Aluminum by electrolysis. This caused aluminum’s price to drop to where it is today (aluminum is still currently refined by this process).
Electrolysis
So, now that we know a little bit about the history of Aluminum, let’s go over how it’s actually produced. As in the introduction, the production of aluminum is made possible by a procedure known as electrolysis. For aluminum, this process is specifically called the Hall–Héroult process, after the names of the chemists that developed it. Out of all the aluminum compounds, the most simple one, Alumina (Aluminum Oxide, Al2O3), is used for this procedure.
Alumina is an ionic compound, meaning that the Al and O don’t exist as atoms but rather as ions, and they each have a charge of 3+ and 2- respectively. The ions’ charges cause an electrostatic interaction between them, making them stick together (positive attracts negative).

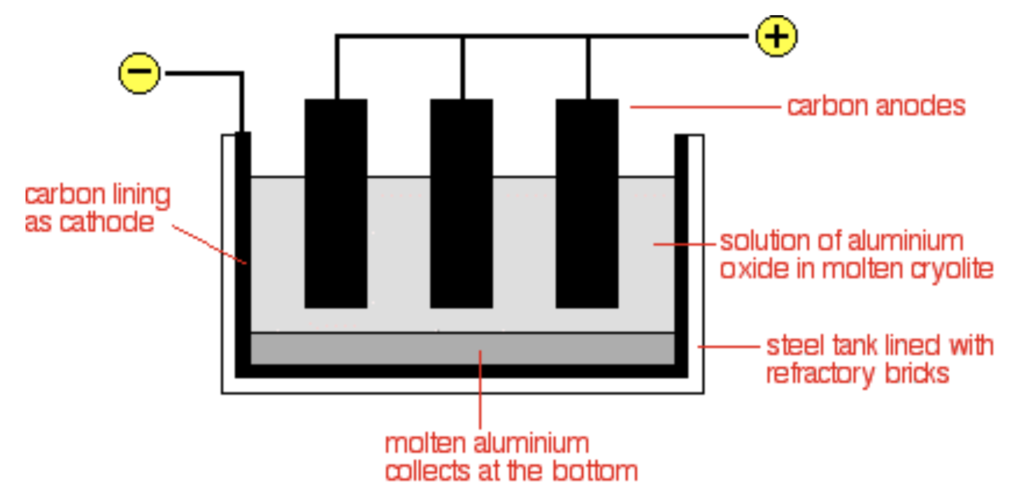
Having that in mind, the big idea of electrolysis is actually pretty simple: you pass a strong electric current through the compound and force the excess electrons in the O2- ions to migrate to the Al3+ ions. The O2- will be oxidized (give off electrons) to form oxygen gas, and the Al3+ will be reduced (take electrons) to form Aluminum metal. The reaction can be written as:

- The aluminum oxide is molten since solid ionic compounds don’t conduct electricity
- The electrons are forced through the liquid, causing the Al3+ to accept them and precipitate as metallic aluminum
[1] [IMAGE] Burns, Ralph A. “Elements, Atoms and the Periodic Table” from Fundamentals of Chemistry. https://wps.prenhall.com/wps/media/objects/439/449969/Media_Portfolio/ch04.html. Last Accessed: 2020/01/27.
[2] [IMAGE] Clark, Jim. “Aluminium”. https://www.chemguide.co.uk/inorganic/extraction/aluminium.html. Last Accessed: 2020/01/27.
[3] [IMAGE] Clemence, Christopher. (2015). “Aluminium Luminaries – Charles Martin Hall”. Aluminium Insider. https://aluminiuminsider.com/aluminium-luminaries-charles-martin-hall/. Last Accessed: 2020/01/27.
[4] [IMAGE] “Alumina Powder (Al2O3)”. Reade. https://www.reade.com/products/alumina-powder-al2o3. Last Accessed: 2020/01/27.
[5] “History of Aluminum”. The Aluminum Association. https://www.aluminum.org/aluminum-advantage/history-aluminum. Last Accessed: 2020/01/27.
[6] Schifman, Jonathan. (2018). “The Entire History of Steel”. Popular Mechanics. https://www.popularmechanics.com/technology/infrastructure/a20722505/history-of-steel/. Last Accessed: 2020/01/27.
[7] Beck, Kevin. (2018). “Where Does Iron Come From or How Is It Made?”. Sciencing. https://sciencing.com/does-iron-come-how-made-4928267.html. Last Accessed: 2020/01/27.
[8] “Charles M. Hall”. Ohio History Central. https://ohiohistorycentral.org/w/Charles_M._Hall. Last Accessed: 2020/01/27.
[9] “Aluminum”. How Products are Made. http://www.madehow.com/Volume-5/Aluminum.html. Last Accessed: 2020/01/27.
Excellent article!
Thank you very much! We appreciate your words a lot. Your article was informative and interesting as well!
Aluminium is my favorite metal
Nice 🙂 Thank you for reading!
Very well written and informative. Thank you.
Thank you very much for reading! We appreciate it!
Great piece
Thanks for your comment! We appreciate it a lot!